Patulin
APS149291- Home
-
Products
-
Analytical Standards
- Additional Standards
- Biomarkers and Metabolomics Standards
- Cell Line Analytical Standards
- Elemental, Ions and Water (Karl Fischer) Standards
-
Environmental
- Additional Organic Reference Materials
- Aqueous Inorganic
- Chemical Weapon Metabolite Standards
- Dioxins & Furans
- Dyes & Metabolites
- Environmental Proficiency Testing
- Flame Retardants
- Hydrocarbons & Petrochemicals
- Mycotoxins
- PCBs & Related Compounds
- Pesticides, Herbicides, and Metabolites
- Pharma & Vet Compounds & Metabolites
- Pharmaceuticals and Personal Care Products (PPCPs)
- Phthalate and Phthalate Metabolite Standards
- Physical Properties
- Polycyclic Aromatic Hydrocarbons (PAHs)
- Priority Pollutant Standard Mixtures
- Priority Pollutant, Endocrine Disruptor, and Chemical Contaminant Standards
- Stable Isotope Labelled Compounds
- Standards for Environmental Regulatory Methods
- Stockholm Treaty Standards
- Volatile Organic Compounds (VOCs)
-
Food & Beverage
- Allergens
- Beverage Reference Materials
- Cannabis-related Compounds
- Dyes & Metabolites
- Environmental Food Contaminants
- Food & Beverage Proficiency Testing
- Food Additives, Flavours & Adulterants
- Food Contact Materials
- Food Processing Contaminants
- Microbiology
- Mycotoxins
- Nutritional Composition Compounds
- Pesticides & Metabolites
- Pharma & Vet Compounds & Metabolites
- Phytochemicals
- Phytochemicalstes
- Stable Isotope Labelled Compounds
- Standards for Food Regulatory Methods
- Food and Cosmetic Component Standards
- Forensic & Toxicology
-
Industrial
- Aluminium Base
- Aqueous Inorganic
- Biodiesel Standards
- Equipment for Sample Preparation
- Laboratory consumables
- Matrix Oils & Solvents
- Metal alloys
- Metallo-organic Standards
- Petroleum Physical Test Standards
- Petroleum Reference Materials
- Process Materials, Geological, Cement & Soils
- Proficiency Testing
- Sulfur, Nitrogen & Chlorine
- XRF Monitor Glasses
- Matrix Materials Standards
- Organic Pollutant Standards
-
Pharmaceuticals, Illicit Drugs & Alcohol
- Additional Drugs
- Amphetamines / Stimulants Standards
- Anesthetics Standards
- Antiasthmatic Drug Standards
- Anticonvulsants / Antiepileptics Standards
- Antidepressants Standards
- Antifungals Standards
- Antihistamines Standards
- API Standards
- Barbiturate Standards
- Benzodiazepines Standards
- British Pharmacopoeia
- Building Blocks
- Cannabinoids Standards
- Carbohydrates
- Cardiac Drug Standards
- Chiral Molecules
- Cocaine Analogs Standards
- COVID-19 Research and Reference Materials
- Cross-linkers
- Elemental Impurities
- Enzyme Activators, Inhibitors & Substrates
- European Pharmacopoeia (Ph. Eur.)
- Excipient Reference Materials
- Extractable and Leachable Reference Materials
- Hallucinogens Standards
- Immunosuppressants
- Impurity Standards
- International Reference Standards for Antibiotics (WHO)
- Neurochemicals
- Nicotine / Tobacco Standards
- Nonbenzodiazepines Drug Standards
- Opiates / Synthetic Analgesic Drug Standards
- P451 Metabolite Standards
- Pharmaceutical Proficiency Testing
- Pharmaceutical Toxicology
- Pharmacopoeial Standards
- Physical Properties
- Reagents According to Pharmacopoeias
- Skeletal Muscle Relaxant Drug Standards
- Sleep Aid Drug Standards
- Stomach Acid Inhibitor Standards
- Physical Properties Standards
- Chromatography Standards
- Ion Sensor Materials
-
Laboratory Supplies
-
3D Printing Materials for Research and Development
- Biodegradeable Polymers
- Bioink
- Ceramics for 3D Printing
- Conductive Inks for 3D Printing
- Hydrogels for 3D Printing
- Metals for 3D Printing
- Monomers and Polymerization Tools for 3D Printing
- Nanomaterials for 3D Printing
- Natural Polymers for 3D Printing
- Organic Semiconductors for 3D Printing
- Oxides for 3D Printing
- Photoinitiator for 3D Printing
- Synthetic Polymers for 3D Printing
- Air-Sensitive Chemistry
- Alternative Energy
- Bioelectronics
- Biomaterials
- Gas Equipment Product Guide
- Micro/NanoElectronics
- Nanomaterials
- Organic and Printed Electronics
- Reference/Calibration Standards
-
3D Printing Materials for Research and Development
- Analytical Reagents
- Derivatization Reagents
- Titration
- Instrumental Analytical Reagents
- LPLC/TLC/Paper Chromatography
- Cell Biology Reagents
- Green Analytical Chemistry
-
Analytical Standards
-
Biological Stains
- Biological Stains and Dyes
-
Biological Stains Kits
- Hematoxylin Stain (Harris, Mayer and Gill)
- H-E Stain (H-E)
- H-E Consistently High Clarity Stain
- Acid Fast Stain (Ziehl-Neelsen)
- Acid Fast Stain (Auramine O)
- Acid Fast Stain (Kinyoun)
- Weak Acid Fast Stain
- Leprosy Bacillus Stain
- Papanicolaou Stain
- Rapid Gram Stain
- Liu's Stain
- Wright-Giemsa Stain
- Giemsa Stain (Blood Cell/Plasmodium)
- Wright Stain
- May-Grunwald's Stain
- Polybrene Test Kit
- Diff-Quik Stain
- Reticulocyte Stain
- Rapid Stain For Female Leukorrhea
- Pathology Hepatin (PAS) Stain
- Alcian Blue Stain (pH 2.5)
- Helicobacter Pylori Stain (Methylene Blue Method)
- Masson's Trichrome Stain
- Oil Red O Stain
- Congo Red Stain
- Elastic Fiber Stain (Victoria Blue Method)
- Van Gieson Stain
- Calcium Salt Stain (Silver Nitrate Method)
- Mucicarmine Stain
- Alcian Blue Stain (pH 1.0)
- Mast Cell Stain (Aldehyde Fuchsin-Orange G Method)
- Mast Cell Stain (Toluidine Blue Method)
- Melanin Stain (Ferrous Sulfate Method)
- Virus Stain (Orcein Method)
- Reticulum Stain
- Nerve Myelin Stain (Luxol Fast Blue Method)
- Tissue Fixative Solution-Bouin's Solution
- Amylase Solution
- ACHE Stain (Copper Ferrocyanide Method)
- Copper Stain (Rhodanine Method)
- Copper Stain (Rubeanic Acid Method)
- Leukocyte Peroxidase (POX) (Oxidation WG-KI) Stain
- Leukocyte Peroxidase Stain (POX) (Benzidine Method)
- Naphthol AS-D Chloroacetate Esterase (AS-DCE) Stain
- Acid Alpha-Naphthyl Acetate Esterase Stain (ANAE)
- α-Naphthyl Acetate Esterase Stain (α-NAE) (Fast Blue Method)
- α-Naphthyl Butyrate Esterase Stain (α-NBE)
- Iron Stain
- Sudan Black B Stain (SBB)
- Neutrophilic Leukocyte Alkaline Phosphatase (NAP) Stain
- Acid Phosphatase (ACP) Stain
- Basophilic Granulocyte Stain (Toluidine Blue)
- Fungal Fluorescence Stain (One-Step Method)
- Fungal Fluorescence Stain (Two-Steps Method)
- Fungal Stain (Hexamine Silver Method)
- Lactic Acid Phenol Cotton Blue Stain
- Bacterial Spore Stain
- Flagella Stain
- Bacterial Capsule Stain
- Cryptococcus Neoformans Stain
- Urinary Sediment Stain (S-M Method) & (S Method)
- Metachromatic Granules Stain (Albert Method)
- Trypan Blue Stain
- Adrenal Chromaffin Cell Stain (Giemsa Method)
- Instruments
- Biological Reagents
- About Us
- Top Sellers
- Recommended Products
-
Resources
- Flyer
- Knowledge Base
-
Research Articles
- Synthesis of Deuterium-Labeled Zaleplon-d5
- The Synergistic Effects of Prostaglandin E1 and Copper on Angiogenesis
- Obeticholic Acid - A Promising Therapy for PBC and NASH
- The Promising Antiviral Potential of Ivermectin
- Unlocking the Secrets of PKAN: 4'-Phosphopantetheine Shows Promise as a Therapeutic Approach
- Blog
- Protocol
- Careers
- Contact








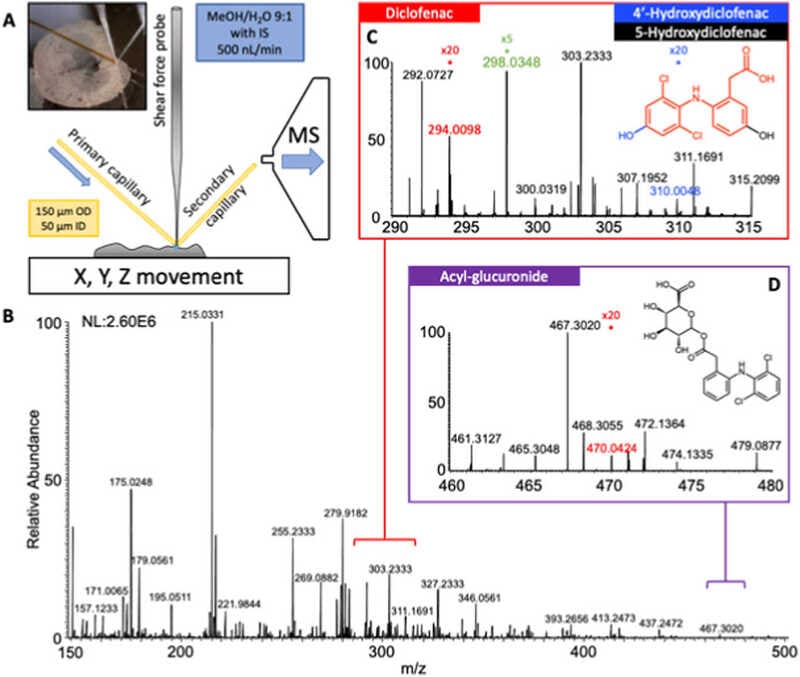 Sanchez, Daniela Mesa, et al. Analytica Chimica Acta 1233 (2022): 340490.
Sanchez, Daniela Mesa, et al. Analytica Chimica Acta 1233 (2022): 340490.
 Ferron, Pierre-Jean, et al. Toxicology in Vitro 33 (2016): 136-146.
Ferron, Pierre-Jean, et al. Toxicology in Vitro 33 (2016): 136-146.
 Szpot, Paweł, Olga Wachełko, and Marcin Zawadzki. Toxics 10.8 (2022): 421.
Szpot, Paweł, Olga Wachełko, and Marcin Zawadzki. Toxics 10.8 (2022): 421.
